The prostate is the largest accessory gland in the male reproductive system.
It secretes proteolytic enzymes into the semen, which act to break down clotting factors in the ejaculate. This allows the semen to remain in a fluid state, moving throughout the female reproductive tract for potential fertilisation.
In this article, we shall look at the anatomy of the prostate – its structure, vasculature and innervation, We shall also consider its clinical correlations.
Anatomical Position
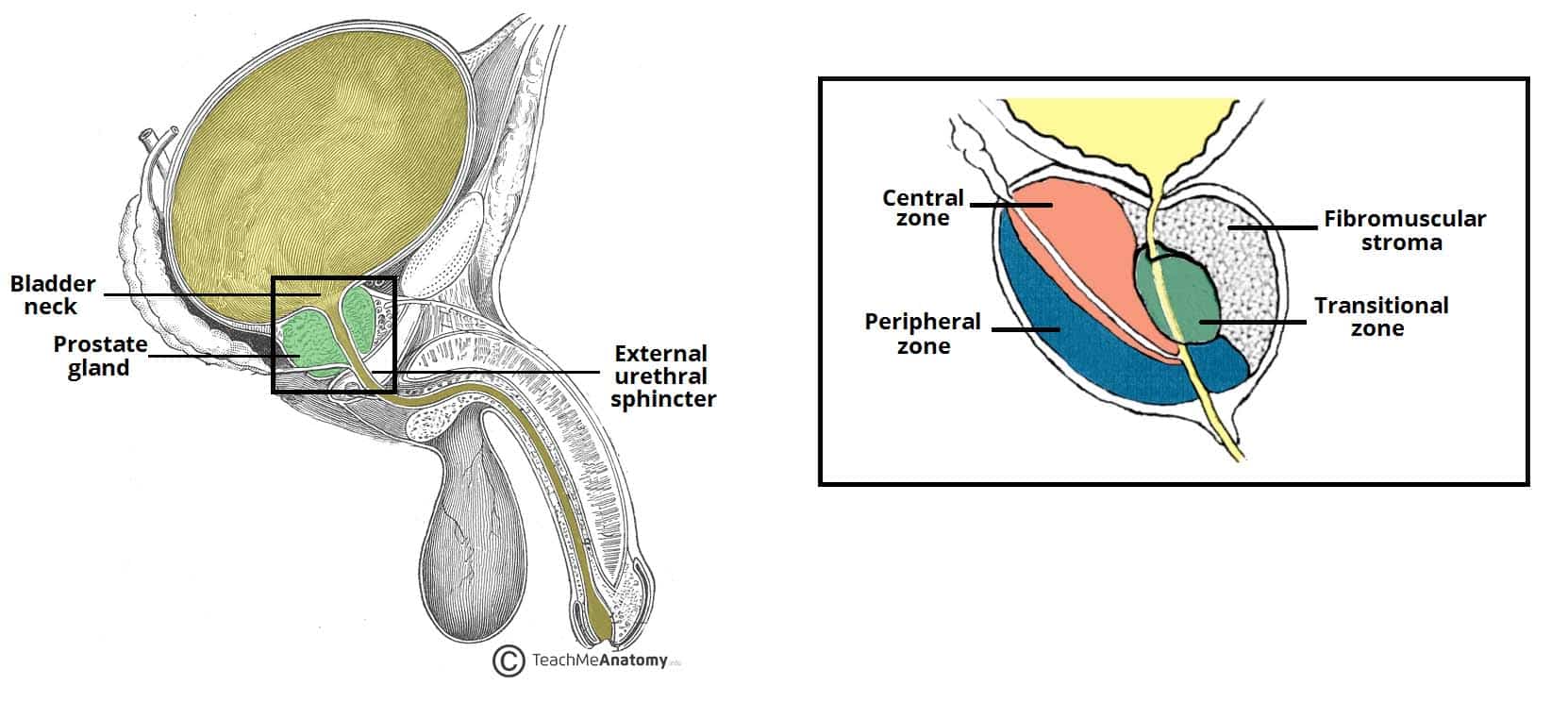
The prostate is positioned inferiorly to the neck of the bladder and superiorly to the external urethral sphincter, with the levator ani muscle lying inferolaterally to the gland.
Most importantly, posteriorly to the prostate lies the ampulla of the rectum – this anatomical arrangement is utilised during Digital Rectal Examinations (DRE), allowing physicians to examine the gland.
The proteolytic enzymes leave the prostate via the prostatic ducts. These open into the prostatic portion of the urethra, through 10-12 openings at each side of the seminal colliculus (or verumontanum); secreting the enzymes into the semen immediately before ejaculation.
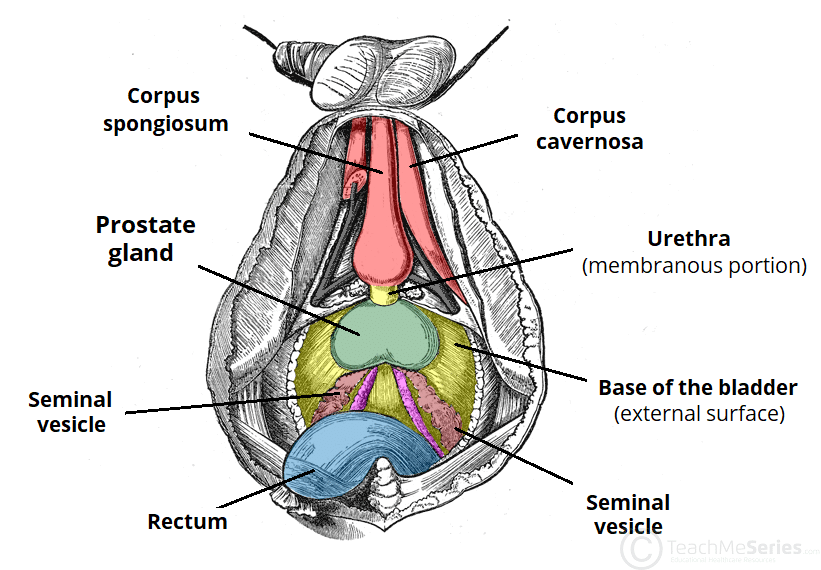
Fig 1 – Inferior view of the structures in the male reproductive system.
Clinical Relevance – Benign Prostatic Hyperplasia (BPH)
Benign prostatic hyperplasia is the increase in size of the prostate, without the presence of malignancy. It is much more common with advancing age, although initial histological evidence of hyperplasia may be evident from much earlier ages (<40 yrs old).
The enlarged prostate may compress the urethra, resulting in symptoms that refer to impaired storage of urine (urinary frequency, urinary urgency, nocturia) and symptoms that refer to impaired voiding (difficulty in initiating micturition, poor stream, intermittent urine stream and terminal dribbling of urine).
BPH is usually caused by hyperplasia of the glands from the transitional zone of the prostate.
Anatomical Structure
The prostate is commonly described as being the size of a walnut. Roughly two-thirds of the prostate is glandular in structure and the remaining third is fibromuscular. The gland itself is surrounded by a thin fibrous capsule of the prostate. This is not a real capsule; it rather resembles the thin connective tissue known as adventitia in the large blood vessels.
Traditionally, the prostate is divided into anatomical lobes (inferoposterior, inferolateral, superomedial, and anteromedial) by the urethra and the ejaculatory ducts as they pass through the organ. However, more important clinically is the histological division of the prostate into three zones (according to McNeal):
- Central zone – surrounds the ejaculatory ducts, comprising approximately 25% of normal prostate volume.
- The ducts of the glands from the central zone are obliquely emptying in the prostatic urethra, thus being rather immune to urine reflux.
- Transitional zone – located centrally and surrounds the urethra, comprising approximately 5-10% of normal prostate volume.
- The glands of the transitional zone are those that typically undergo benign hyperplasia (BPH)
- Peripheral zone – makes up the main body of the gland (approximately 65%) and is located posteriorly.
- The ducts of the glands from the peripheral zone are vertically emptying in the prostatic urethra; that may explain the tendency of these glands to permit urine reflux.
- That also explains the high incidence of acute and chronic inflammation found in these compartments, a fact that may be linked to the high incidence of prostate carcinoma at the peripheral zone.
- The peripheral zone is mainly the area felt against the rectum on DRE, which is of irreplaceable value.
The fibromuscular stroma (or fourth zone for some) is situated anteriorly in the gland. It merges with the tissue of the urogenital diaphragm. This part of the gland is actually the result of interaction of the prostate gland budding around the urethra during prostate embryogenesis and the common horseshoe-like muscle precursor of the smooth and striated muscle that will eventually form the internal and external urethra sphincter.
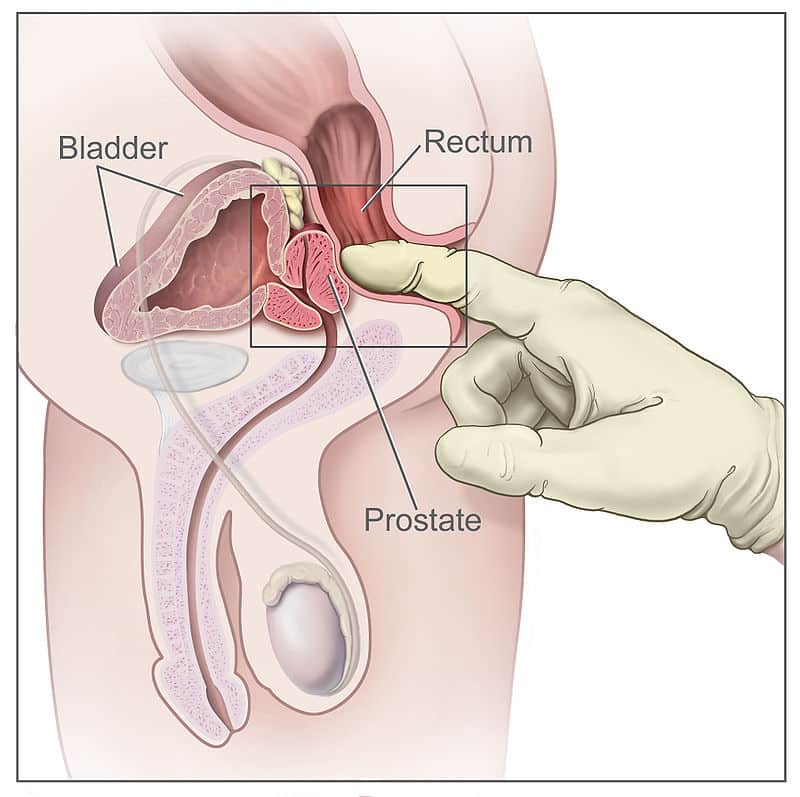
Fig 3 – The prostate can be felt anteriorly during a digital rectal examination. The peripheral zone is mainly palpated.
Vasculature
The arterial supply to the prostate comes from the prostatic arteries, which are mainly derived from the internal iliac arteries. Some branches may also arise from the internal pudendal and middle rectal arteries.
Venous drainage of the prostate is via the prostatic venous plexus, draining into the internal iliac veins. However, the prostatic venous plexus also connects posteriorly by networks of veins, including the Batson venous plexus, to the internal vertebral venous plexus.
Innervation
The prostate receives sympathetic, parasympathetic and sensory innervation from the inferior hypogastric plexus. The smooth muscle of the prostate gland is innervated by sympathetic fibres, which activate during ejaculation.
Neurovascular Bundles
The prostate is flanked by the two neurovascular bundles that travel through the pelvic floor towards the penis, supplying it with nerve fibres and blood vessels for the corpora cavernosa. The integrity of these bundles is critical for normal erection.
During surgery for prostate cancer (radical prostatectomy), damage is often inevitable to one or both of these bundles, resulting in impairment of erectile function. Special nerve-sparing techniques may prevent extensive damage to these bundles, thus allowing for post-operative potency.
Clinical Relevance – Prostatic Carcinoma
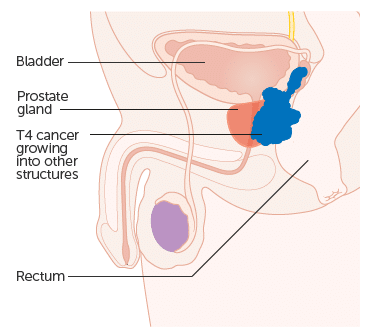
Prostatic carcinoma represents the most commonly diagnosed cancer in men, especially in countries with high sociodemographic index. The malignant cells commonly originate from the peripheral zone, although carcinomas may arise (more rarely) from the central and transition zones too. It is still debatable that the latter tumors may present with lower malignant potential.
However the proximity of the peripheral zone to the neurovascular bundle that surrounds the prostate may facilitate spread along perineural and lymphatic pathways, thus increasing the metastatic potential of these tumors. Malignant cells may invade adjacent structures (bladder, seminal vesicles) and/ or lymphatic and blood vessel routes to give distant metastases. Prostate carcinoma also commonly spreads via the Batson venous plexus to the vertebral bodies and cause skeletal metastases.
A DRE may reveal a hard, irregular prostate gland. In most cases the serum PSA values will be increased. However, due to the peripherally-advancing tumor, symptoms may be minimal, as obstruction occurs usually at late stages. One should also keep in mind that the high incidence of prostate carcinoma is found in elderly men, who may already have symptoms due to BPH.
Prostate Specific Antigen
The Prostate Specific Antigen (PSA) is an enzyme (serine protease) secreted by the prostatic epithelium that aids the liquification of the ejaculate by lysing seminal vesicle proteins. However the main clinical use of PSA is as a tumor marker specific for prostate carcinoma.
Increased levels of serum PSA may suggest the presence of prostate cancer. Nonetheless other conditions that may “irritate” the prostate (such as inflammation, severe constipation, extended sexual intercourse or catheterisation) may also increase the PSA levels in the serum.